Mathematical modelling of clot groth
Mathematical modeling of clot growth (thrombus formation) aims to understand the complex interactions that drive the process of blood clotting. This involves modeling the dynamics of platelets, coagulation factors, blood flow, and the interactions between them. Models can help predict clot behavior under different physiological conditions, design treatments for clot-related disorders, and provide insights into the underlying mechanisms.
Key Components in Clot Growth Modeling
-
Platelet Adhesion and Aggregation:
- Platelet activation: Platelets adhere to the site of injury and become activated, releasing substances like ADP and thromboxane A2 (TXA2) that promote further aggregation.
- Aggregation: Platelets aggregate, forming the initial plug, mediated by fibrinogen binding to GPIIb/IIIa receptors.
-
Coagulation Cascade:
- The coagulation cascade is a chain reaction involving several enzymes and cofactors that result in the conversion of fibrinogen to fibrin, which stabilizes the platelet plug.
- Thrombin generation plays a central role in this cascade, activating more platelets and converting fibrinogen to fibrin.
-
Fibrin Network Formation:
- Fibrin fibers are cross-linked by Factor XIII, forming a mesh that stabilizes the growing clot.
- The balance between clot growth and degradation (fibrinolysis) is crucial to prevent excessive clotting.
-
Hemodynamics:
- Blood flow influences clot formation by transporting clotting factors, platelets, and thrombin.
- Shear forces: High shear rates (in arteries) can enhance platelet adhesion, while low shear rates (in veins) favor the coagulation cascade.
- Models often use the Navier-Stokes equations to simulate the fluid dynamics of blood flow around the clot.
Mathematical Approaches to Modeling
-
Continuum Models:
- Use partial differential equations (PDEs) to describe concentrations of clotting factors, thrombin, platelets, and fibrin over time and space.
- Equations account for diffusion, advection (transport by blood flow), and reaction terms representing biochemical interactions.
- Example: The reaction-diffusion-advection equation models the propagation of thrombin and the spread of the clot.
Example of PDE for thrombin concentration:
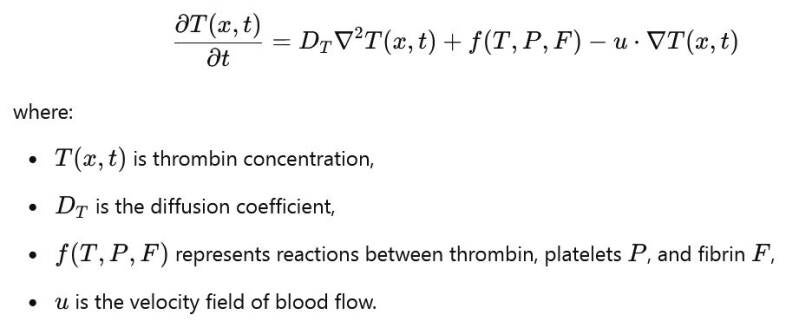
-
Agent-Based Models (ABMs):
- Treat individual platelets and clotting factors as "agents" with specific rules for behavior (e.g., adhesion, activation, and aggregation).
- ABMs can simulate detailed interactions and the stochastic nature of platelet aggregation and clot formation.
- Useful for capturing heterogeneities in clot growth and incorporating mechanical properties of clots.
-
Stochastic Models:
- Capture the randomness inherent in the molecular interactions of clotting factors.
- Monte Carlo simulations are often used to account for the probabilistic nature of biochemical reactions and platelet collisions.
-
Hybrid Models:
- Combine continuum and agent-based models to take advantage of both approaches.
- For example, a continuum model could be used for the diffusion of thrombin, while an agent-based model captures individual platelet behavior.
Boundary Conditions and Geometry
- Domain Geometry: Clots form in specific vascular geometries (e.g., in a cylindrical blood vessel). Realistic 3D geometries can be incorporated using numerical methods such as finite element methods (FEM) or computational fluid dynamics (CFD) tools.
- Boundary Conditions: The boundary where the vessel is injured requires special attention. Conditions must describe platelet adhesion, thrombin activation, and fibrin generation at the injury site.
Modeling Challenges
-
Multiscale Complexity:
- Clot formation occurs across multiple scales: molecular (clotting factors), cellular (platelets), and macroscopic (blood flow).
- Integrating different scales in a single model is complex.
-
Nonlinear Dynamics:
- Clotting involves nonlinear feedback loops, such as thrombin amplification, which makes predicting clot behavior difficult.
-
Parameter Estimation:
- Accurate models require detailed knowledge of parameters like reaction rates, diffusion coefficients, and flow conditions, many of which are difficult to measure.
-
Thrombosis and Embolism:
- Understanding how clots grow excessively (thrombosis) or detach and travel (embolism) is critical in predicting pathological conditions.
Applications of Clot Growth Models
-
Disease Prediction:
- Models help in understanding conditions like deep vein thrombosis (DVT), arterial thrombosis, and disseminated intravascular coagulation (DIC).
-
Drug Development:
- Testing the effect of anticoagulant therapies (e.g., heparin, warfarin) on clot formation and stability in silico.
-
Personalized Medicine:
- Incorporating patient-specific data, such as blood flow profiles and clotting factor levels, to predict individualized responses to treatments.
-
Medical Device Design:
- Simulating how blood clots form around medical devices like stents or heart valves, helping improve design to minimize thrombosis risk.
By combining detailed biological knowledge with mathematical techniques, models of clot growth offer powerful tools for exploring normal and pathological clotting processes.